Which Best Describes the Law of Conservation of Mass
When a substance transforms from a liquid state to a gaseous state it loses mass. When reactants contain both a solid and a liquid the solid counts toward the overall mass and.
What Is The Law Of Conservation Of Mass About Quora
So the mass of the product equals the mass of the reactant.

. Products in the form of gases are not considered a part of the total mass change from reactants to products. Lead Salt Car Chair all of the. The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations.
10 grams of reactant 10 grams of products. 5 How do you describe a food chain. Represent this reaction in terms of law of conservation of mass.
Mass is neither created nor destroyed in a chemical reaction. Matter can be converted to more massive things. The statement that best describes the Law of Conservation of Mass is when a physical or a chemical change occurs matter is not created or destroyed.
Mass of reactants Mass of products. A sample of N 2. This law was later amended by Einstein in the law of conservation of mass-energy which describes the fact that the total mass and energy in a system remain.
6 How does the food chain and food web help in flow of energy in. Simply stated the law of conservation of mass means matter cannot be created or destroyed but it can change forms. Hence it is proved that the law of conservation of mass is followed by the above reaction.
A cannot be created but can be destroyed. Which statement best describes the law of conservation of mass. Click to read full detail here.
1Suppose 10 atoms of carbon C react with 20 atoms of oxygen O. Log in for more information. In chemistry the law is used to balance chemical equations.
The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses such the amount of gas consumed or produced during a reaction. 10 gram of CaCO 3 38 grams of CO 2 62 grams of CaO. The Law of Conservation of Mass.
Question 1 The law of conservation of mass states that matter _____. For example when wood burns the mass of the soot ashes and gases equals the original mass of the charcoal and the oxygen when it first reacted. This law postulates that the mass is not created or destroyed only transformed This means that the reagents interact with each other and form new products with physical and chemical properties different from those of the reagents because.
Matter can be converted to less massive things. Which of the following can be classified as matter. Which term best fits the following definition.
According to the law of conservation of mass the mass of the products in a chemical reaction must equal the mass of the reactants. Which best describes the law of conservation of mass. Products in the form of gases are not considered a part of the total mass change from reactants to products.
Must be less than 640g b. The equation below shows a general equation for a reaction and the amounts of the substance are written underneath. Must be more than 640g d.
You might be interested in. According to law of conservation of mass. According to the law of conservation of mass the mass of the products in a chemical reaction must equal the mass of the reactants.
Mass is conserved in a chemical reaction but not during a physical change. The number and type of atoms must be the same for both reactants and products. 1 Describe How A Food Chain Follows The Law Of Conservation Of Energy.
In a chemical reaction 300 grams of reactant A are combined with 100 grams of. A theory before it has become well established. According to the law of conservation of mass if the products of a reaction have a mass of 640g then the total mass of the reactants a.
The statement that best describes the Law of Conservation of Mass is when a physical or a chemical change occurs matter is not created or destroyed. Must be equal to 640g c. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
The mass of the reactants and products is equal and is not dependent on the physical state of the substances. 3 How is the law of conservation of mass demonstrated in biological systems such as food chains K. What is stated by the law of conservation of mass.
Definition Equation Examples. This answer has been confirmed as correct and helpful. The coefficients in front of the chemicals in the reactants should be based on the physical state of the products.
B cannot be created or destroyed. 4 What are the 4 steps of the food chain. Added 8222020 82556 AM.
Added 6302015 63003 AM. Asked Sep 21 2016 in Chemistry by Annha. 2 How does energy move through a food chain.
Which of the following statements best explains the law of conservation of mass. Log in for more information. C can be created and.
The Law of Conservation of Matter is also called the law of conservation of mass or the Law of Lomonósov-Lavoisier. Credit for discovering the law may be given to. The coefficients in front of the chemicals in the reactants should be based on the physical state of the products.
Which best describes the law of conservation of mass. All matter contains the same mass. Which of the following statements best describes the Law of Conservation of Mass.
Which of the following statements best describes the Law of Conservation of Mass. The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. Which best describes the law of conservation of mass.
100 g of water is heated to give steam. Can anyone explain how does the balanced chemical equation show the law of conservation of mass. Is not related to the mass of the.

Law Of Conservation Of Mass Energy The Carbon Cycle Ppt Download

Law Of Conservation Of Mass Example Youtube

The Carbon Cycle Https Www Youtube Com Watch V Nzimo8ksxiu In 2021 Carbon Cycle Photosynthesis And Cellular Respiration Conservation Of Mass

Describe An Experiment Which Proves The Law Of Conservation Of Mass Brainly In

The Law Of Conservation Of Mass Can Be Demonstrated By A Chemical Reaction Which Of The Following Brainly Com

Which Of The Following Models Best Demonstrates The Law Of Conservation Of Mass Look At The Brainly Com
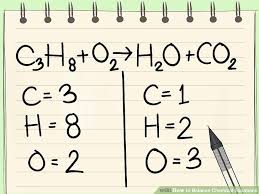
8 P 1 4 Law Of Conservation Of Mass Quiz Quizizz
What Is The Law Of Conservation Of Mass About Quora

Engenharia Ou Arte Parte 2 O Professor Que Transforma Calculos Em Obras De Arte Learn Physics Physics Lessons Physics Teacher

The Law Of Conservation Of Mass Can Be Demonstrated By A Chemical Reaction Which Of The Following Brainly Com

Pin On Concise Chemistry Class 7

Synthesis Physical Vs Chemical Properties Changes Physical Vs Chemical Properties Physics Teaching Chemistry

Atoms And Atomic Structure Task Cards Task Cards Atomic Theory Atomic Structure

Law Of Conservation Of Mass Worksheet Conservation Of Mass Worksheets Homework Assignments

Using An Argument Driven Inquiry Framework For Student Exploration Of Gas Laws Chemical Educatio Computational Thinking Chemical Education Algebraic Thinking

Chemistry Flip Notes Percent Composition Empirical And Molecular Formulas Molecular Chemistry Formula

Comments
Post a Comment